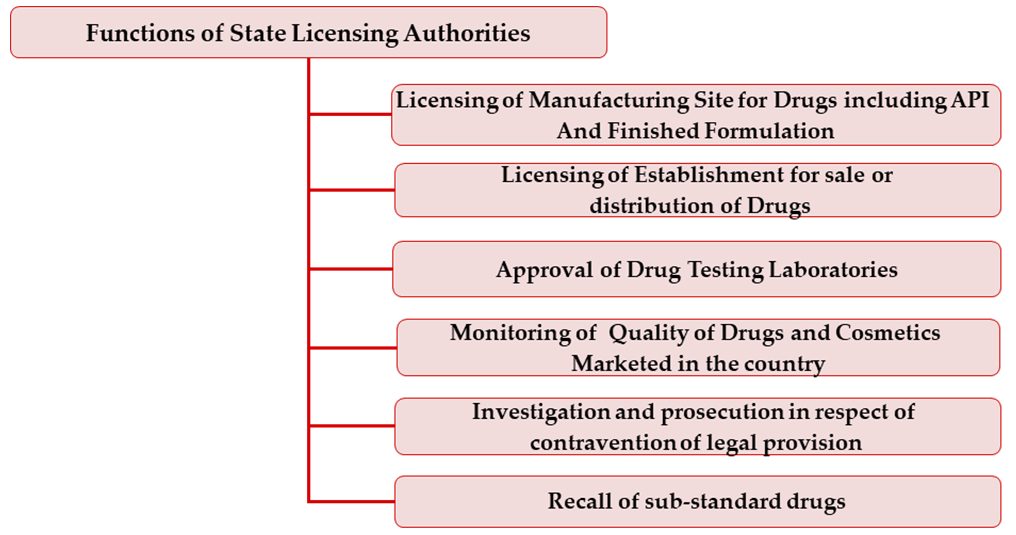
Principle
The principle on which the Drugs & Cosmetics Act function is by a system of licensing under which all the activities involved in manufacture, sale and distribution of Drugs & Cosmetics are controlled.
Drug regulatory system in India
Drug is in concurrent list of Indian Constitution. It is governed by both Centre and State Governments under the Drugs & Cosmetics Act 1940 & Rules 1945 there under.
The mission of regulatory authorities is to promote and protect public health. The lack of access to medicines remains a huge concern, whether these are essential medicines, vaccines, orphan drugs or drugs for tropical diseases.
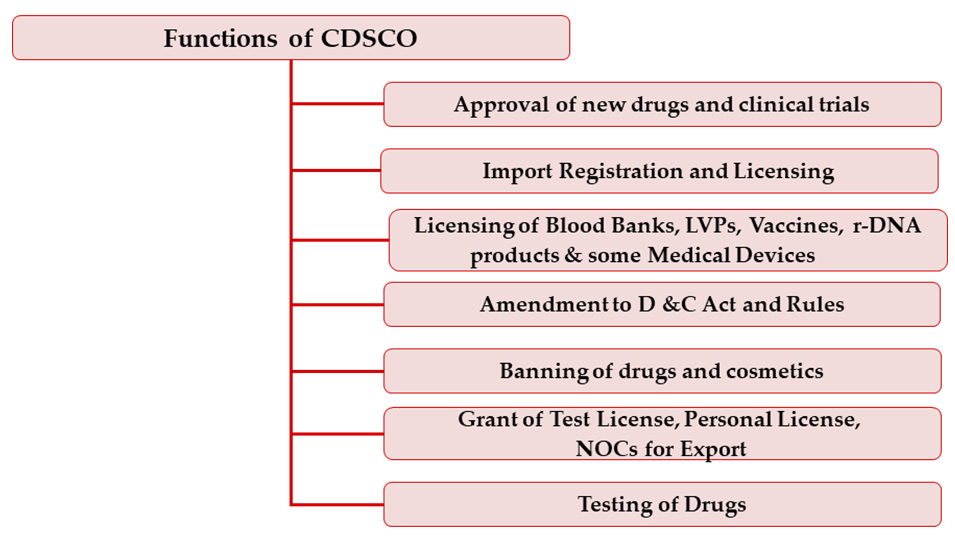
To facilitate access, regulators and all other stakeholders need to be actively involved in identifying difficulties and seeking solutions leading to balanced approaches to access which do not compromise public health safeguards.
Regulators have a role and responsibility to facilitate access to drugs of public health importance including proposing changes to the respective regulations in order to facilitate access without compromising quality, safety and efficacy.
As part of the medicines approval process, regulators should carry out an appropriate risk benefit assessment to allow for adjustment to the needs and profile of the anticipated patient populations.