Medical devices are being widely used in all branches of medicines, surgery and community not only in India but across the globe. Keeping in view the broad objectives for ensuring protection of the health & safety of patients, healthcare professionals and others, the Ministry of Health & Family Welfare, Government of India has released the Medical Device Rules, 2017, effective from 1st January, 2018 for regulating Medical Devices being used in the country. As India is playing a major role in marketing of these devices in Asia, and beyond, regulating Medical Devices poses a real challenge, upon implementation of the Medical Device Rules, 2017 which ultimately aim at replacing the existing Rules of the Drugs and Cosmetics Act, 1940.
Regulation of Medical devices in India:
The Central Drugs Standard Control Organization (CDSCO) under Directorate General of Health Services in Ministry of Health and Family Welfare (MoHFW), Government of India (GoI) is the National Regulatory Authority (NRA) responsible for approval of manufacturing, import, conduct of clinical trials, laying down standards, sale and distribution of medical devices through enforcement and implementation of the Medical Devices Rules, 2017 released through Gazette of India notification G.S.R. 78(E), dated 31st January 2017 by the MoHFW, GoI.
Central Licensing Authority
The Drugs Controller General of India (DCGI) shall be the Central Licensing Authority, competent for the enforcement of these rules in matters relating to,-
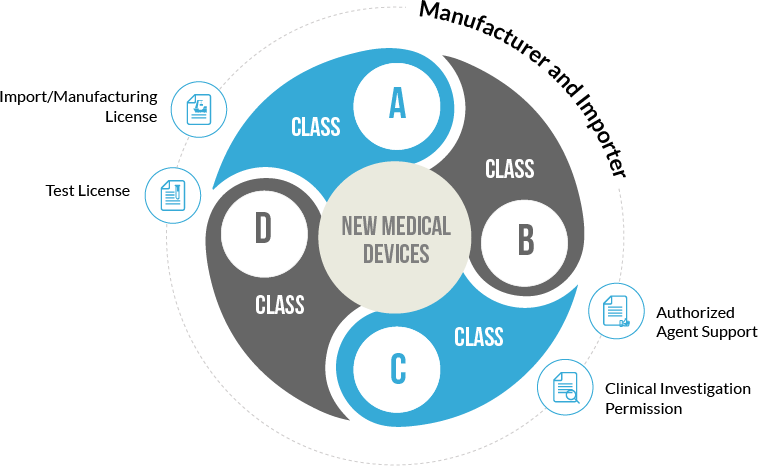
- Import of all Classes of medical devices
- Manufacture of Class C and Class D medical devices
- Clinical investigation and approval of investigational medical devices
- Clinical performance evaluation and approval of new in vitro diagnostic medical devices
- Co-ordination with the State Licensing Authorities.
State Licensing Authority
State Licensing Authority The State Drugs Controller, by whatever name called, shall be the State Licensing Authority and shall be the competent authority for enforcement of these rules in matters relating to,
- Manufacture for sale or distribution of Class A or Class B medical devices
- Sale, stock, exhibit or offer for sale or distribution of medical devices of all classes.
